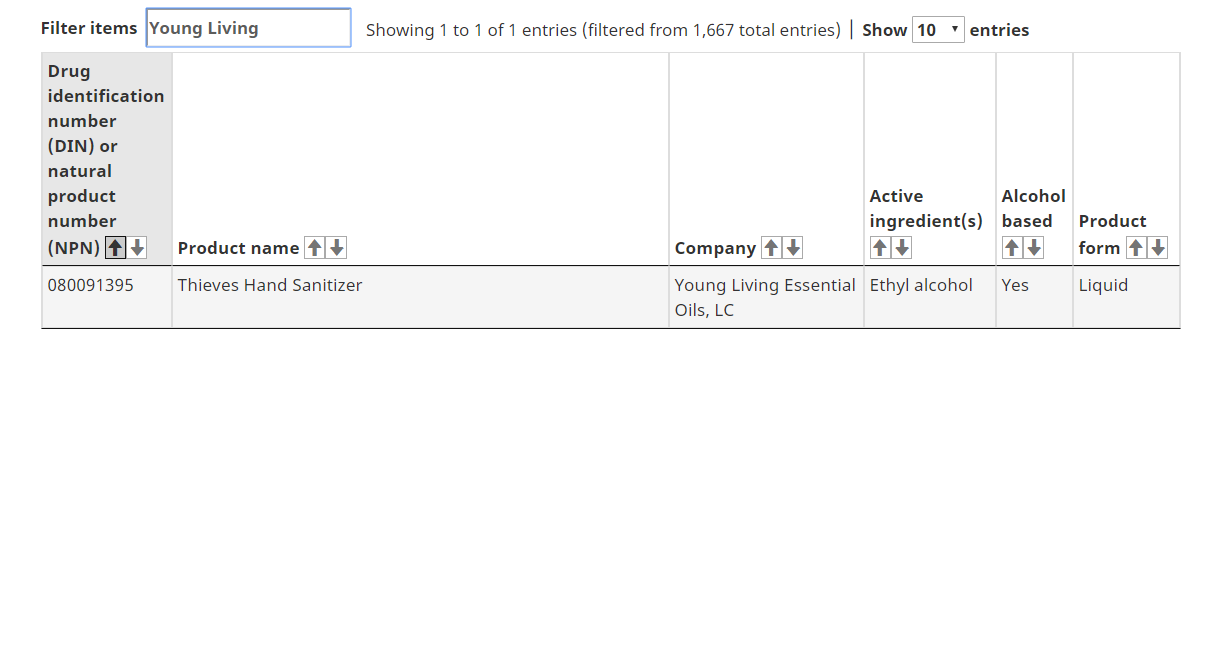
The following antiseptic/antibacterial skin cleansers or hand sanitizers meet Health Canada's requirements for safety, effectiveness and quality.
How to find out which antiseptic skin cleansers or hand sanitizers meet Health Canada's requirements
Locate the Natural Product Number (NPN) or Drug Identification Number (DIN) on the product label
Look for that number on the hand sanitizers list
Like all hand sanitizers, these products are not to be ingested and should be kept out of reach of children. Alcohol-based hand sanitizers must carry/include the following warnings on the label:
For external use only.
When using this product avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Stop use and ask/consult a doctor/physician/health care practitioner/health care provider/health care professional if irritation develops.
Keep out of reach of children. If swallowed, call a poison control centre or get medical help right away.
Flammability warning: Keep away from open flame and sources of heat.
As with all drug products, Health Canada recommends that users always follow the directions for use on the product label.
An expedited access process is in place for applicants who wish to seek the necessary authorizations from Health Canada to manufacture and sell alcohol-based hand sanitizers. For more information, please contact the Natural and Non-prescription Health Products Directorate at hc.nnhpd-dpsnso.sc@canada.ca.
You can also search our databases for more information:
Licensed Natural Health Products Database for natural health products
Drug Product Database for drug products
Get more information on natural health products and drug products.
Access the Guide on Health Canada's interim expedited licensing approach for alcohol-based hand sanitizers.
Read Health Canada's decision to temporarily allow technical grade ethanol in the manufacture of hand sanitizers:
Notice to industry
Suppliers of technical grade ethanol for use in the production of hand sanitizers
Risk assessment summary report